

A well-functioning health system ensures equitable access and use of essential medical products, vaccines and technologies with a high standard of quality, safety, the efficacy that are cost- effective1. Essential drugs2 should be available at all times, in adequate amounts, in the appropriate dosage, with assured quality, and at an affordable price1.
The availability of quality essential drugs at public healthcare facilities reduces out-of-pocket expenditure (OoPE) and enhances the community’s confidence in the public health system. A lack of it drives people towards the private sector3. In India, ~60-70% of health care is catered to by the private sector4 leading to high OoPE. However, even those seeking care in public health facilities incur significant OoPE due to drugs, accounting for over 80% and 50% of OoPE in outpatient and inpatient care, respectively5. Moreover, most private and public6 insurance packages do not cover outpatient expenses.
The availability of quality essential drugs at public healthcare facilities reduces out-of-pocket expenditure (OoPE) and enhances the community’s confidence in the public health system. A lack of it drives people towards the private sector3. In India, ~60-70% of health care is catered to by the private sector4 leading to high OoPE. However, even those seeking care in public health facilities incur significant OoPE due to drugs, accounting for over 80% and 50% of OoPE in outpatient and inpatient care, respectively5. Moreover, most private and public6 insurance packages do not cover outpatient expenses.
The Performance Audit Report by the Comptroller and Auditor General (CAG) of India for public health facilities in Uttar Pradesh has highlighted some of the critical challenges in the public health drug supply chain7.
Figure 1 explains the legacy public health supply chain model in UP.
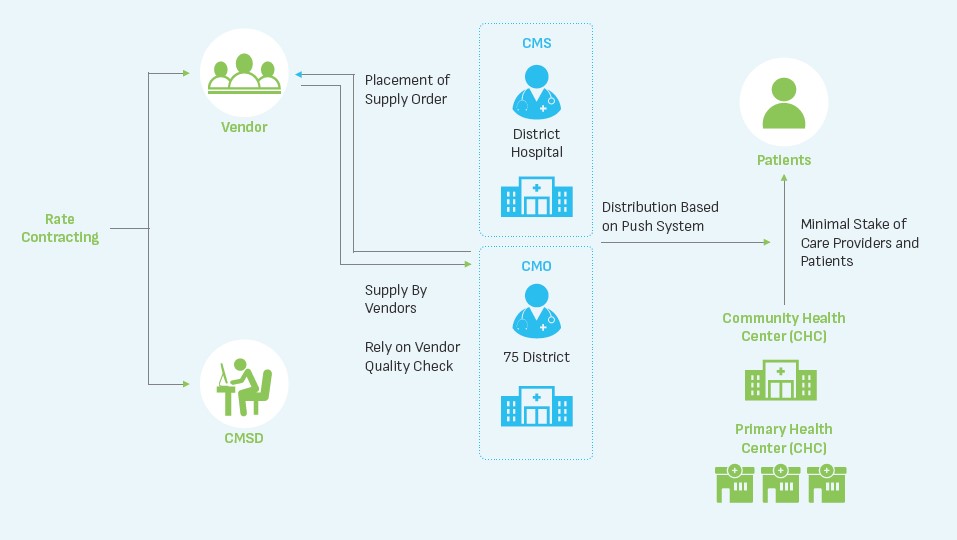
The key stakeholders as per the legacy model were State-level intradepartmental body (within Directorate of Health Services) named “Chief Medical Store Depot (CMSD)”, District Chief Medical Officers / Chief Medical Superintendents Suppliers. The two most important stakeholders, namely the care providers and the patients, were mere recipients of the ‘push model’ with no influence on the availability of drugs directly or indirectly. The audit report noted that the weak supply chain for procurement of essential drugs potentially exposed patients to financial hardships and diminished public trust in the health system. A serious policy response was recommended to address gaps in drug and equipment procurement in Uttar Pradesh.
This necessitated a thorough review of the then existing public health drug supply chain system model, studying best practices in other states countries redesigning for ensuring availability of essential drugs required for the patients at all public health facilities.
Government of Uttar Pradesh, with its firm commitment to ensure quality drugs at all levels of the public health facilities and with support from the Uttar Pradesh Technical Support Unit (UP TSU), a unit implemented by India Health Action Trust (IHAT) in partnership with the University of Manitoba, Canada, studied public health supply chain models deployed across various states in India to identify an efficient solution for UP’s public health supply chain.
It was recommended by UPTSU to have a separate company for procurement of drugs and equipment required for public health facilities replacing the intradepartmental body (CMSD), which was duly accepted. Accordingly, in March 2018, the Uttar Pradesh Medical Supplies Corporation (UPMSC) was incorporated as an independent corporation with clearly defined goals to undertake centralised procurement and management of essential drugs, equipment and medical supplies.
UPTSU further supported the Government of Uttar Pradesh (GoUP) in operationalising UPMSC by developing Articles of Association (AoA) and Memorandum of Association (MoA) that defined the organisational structure, recruitment of human resources, development of policies and standard operating procedures, identification and operationalisation of warehouses. In addition, UPTSU helped in the customisation and rollout of the Drugs and Vaccine Distribution Management System (DVDMS) – which serves as the IT backbone for managing the supply chain of drugs in the state. UPTSU continues to support GoUP in strengthening UPMSC to be responsive to the needs of the public health supply chain system.
Best practices from various states were adapted to design the model that has fewer hierarchy levels, leaner teams and streamlined processes (including digitisation), leading to a transparent, efficient and responsive system.
The overview of the redesigned model and its essential features are shown in Figure 2 below.
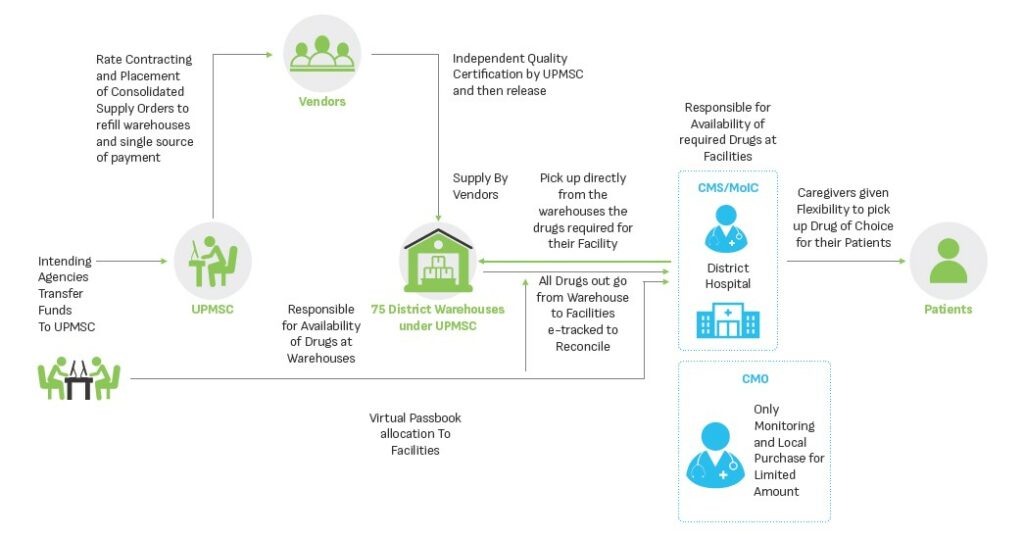
The sustenance and effectiveness of UPMSC are contingent on continuous improvement of the processes to adapt and respond to the needs of the public health supply chain while ensuring a continuous supply of quality essential drugs and equipment to the health facilities. Best practices from successful supply chain models in the country were referred to identify six pillars of responsive and efficient supply chain process. These pillars, depicted below, have been adapted to UP’s context and provide the foundation for the functioning of Uttar Pradesh Medical Supplies Corporation.
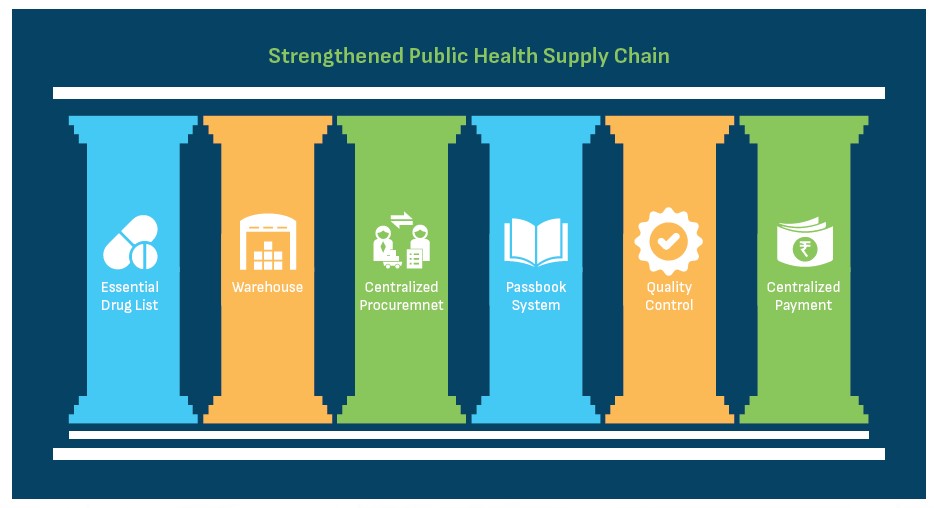
(i) Essential Drug List (EDL): Essential drug list includes those generic drugs which are necessary to satisfy the priority healthcare needs of the majority of the state population. In UP, the Essential Drug List has been pruned down from ~1300 to ~300 items. Initial demand was forecasted based on end-user consumption, disease patterns and demography of the state. Only the drugs that are consumed are replenished, leading to a consumption-based ordering which ensures that the drug budget is utilised on drugs that are actually being consumed.
(ii) Warehouses: District Warehouses are critical cogs of the public health supply chain system where drugs are stored. Warehouses with adequate storage space and climate conditions for drugs and equipment have been established in each district. UPMSC is responsible for maintaining a 24 * 7 supply of all essential drugs at the warehouses.
(iii) Centralised Procurement: UPMSC conducts centralised procurement of drugs through rate contracting, scientific forecasting and past consumption analysis and issuance of purchase orders. This helps in the generation of economies of scale, reduction in costs and maintenance of uniformity of drug availability across the state.
(iv) The Passbook System: A passbook system has been established with notional budgets allotted to each facility. The passbook is not a financial tool as it works on a notional budget but acts as a record-keeping tool. Each facility is responsible to pick up supplies as per their requirement within the budget allocated. The passbook system ensures that budget is being spent on drugs that are being consumed, provides visibility on drug consumption patterns among health facilities, facilitates rational budget usage, links high footfall facilities with drug usage, forecasts drug requirements and prevents pilferage due to the double-entry system.
(v) Quality Control: Each batch of drugs supplied at warehouses is tested by empanelled NABL accredited laboratories to ensure the quality of the drugs. A “Double Blind” method is followed to ensure no single party knows details about the batch or the laboratory where the sample has been sent for testing. The blinding process involves removing the identifiers that can link the sample to the manufacturer or warehouses and adds a system-generated code. The drugs under testing are kept under the “quarantine” area and are released to public health facilities only after successful testing by the laboratories.
(vi) Centralised Payment: Centralised payments are being made in a timely manner to the vendors so that they are not required to follow up with the district level authorities for release of their payment. This encourages drug vendors with large capacity of manufacturing drugs to participate in the procurement process.
Availability of essential drugs has improved after the establishment of UPMSC. There is an increasing trend observed in drug availability since UPMSC started the supplies, as shown in the figure below.
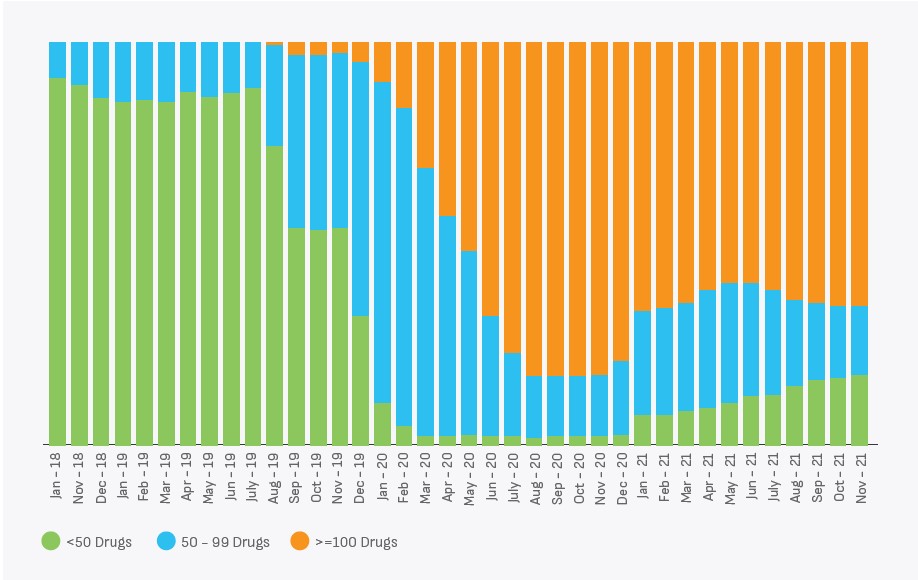
UP TSU continues to provide technical support to GoUP to strengthen the public health procurement system in Uttar Pradesh. The focus is to strengthen the six pillars of the public health supply chain to facilitate the provision of an uninterrupted supply of essential drugs and medical supplies to the facilities. Processes need to be developed to periodically review the essential drugs list to ensure that the list caters to the public health priorities of the state. The-state-of-the art warehouses will be established across all 75 districts of the state. The use of passbook system has to be saturated across all public health facilities. Demand estimation has to be consumption based and a centralised payment system has to be streamlined to ensure minimum lead time for payment.
1 Monitoring the building blocks of health systems: A handbook of indicators and their measurement strategies
2 World Health Organization (WHO) defines essential drugs or medicines as World Health Organization (WHO) defines essential drugs or medicines as “those that satisfy the priority health care needs of the population”.
3 As noted in the context of unavailability of surgical services by the Performance Audit Report of the Comptroller and Auditor General of India on Hospital Management in Uttar Pradesh, dated 2019
4 Including pan India Pradhan Mantri Jan Arogya Yojana (PM-JAY)
5 http://mospi.nic.in/sites/default/files/publication_reports/KI_Health_75th_Final.pdf
6 Performance Audit Report of the Comptroller and Auditor General of India Hospital Management in Uttar Pradesh, Government of Uttar Pradesh, 2019
7 Performance Audit Report of the Comptroller and Auditor General of India Hospital Management in Uttar Pradesh, Government of Uttar Pradesh, 2019